Peptides are a class of compounds formed by the connection of multiple amino acids through peptide bonds. They are ubiquitous in living organisms. Up to now, tens of thousands of peptides have been found in living organisms. Peptides play an important role in regulating the functional activities of various systems, organs, tissues and cells and in life activities, and are often used in functional analysis, antibody research, drug development and other fields. With the development of biotechnology and peptide synthesis technology, more and more peptide drugs have been developed and applied in clinic.
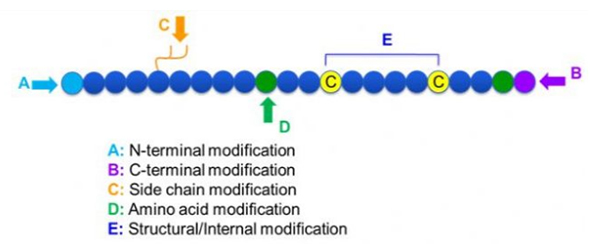
There are a wide variety of peptide modifications, which can be simply divided into post modification and process modification (using derived amino acid modification), and N-terminal modification, C-terminal modification, side chain modification, amino acid modification, skeleton modification, etc., depending on the modification site (Figure 1). As an important means to change the main chain structure or side chain groups of peptide chains, peptide modification can effectively change the physical and chemical properties of peptide compounds, increase water solubility, prolong the action time in vivo, change their biological distribution, eliminate immunogenicity, reduce toxic side effects, etc. In this paper, several major peptide modification strategies and their characteristics are introduced.
1. Cyclization
Cyclic peptides have many applications in biomedicine, and many natural peptides with biological activity are cyclic peptides. Because cyclic peptides tend to be more rigid than linear peptides, they are extremely resistant to the digestive system, can survive in the digestive tract, and exhibit a stronger affinity for target receptors. Cyclization is the most direct way to synthesize cyclic peptides, especially for peptides with large structural skeleton. According to the cyclization mode, it can be divided into side chain-side chain type, terminal - side chain type, terminal - terminal type (end to end type).
(1) sidechain-to-sidechain
The most common type of side-chain to side-chain cyclization is disulfide bridging between cysteine residues. This cyclization is introduced by a pair of cysteine residues being deprotected and then oxidized to form disulfide bonds. Polycyclic synthesis can be achieved by selective removal of sulfhydryl protection groups. Cyclization can be done either in a post-dissociation solvent or on a pre-dissociation resin. Cyclization on resins may be less effective than solvent cyclization because the peptides on resins do not readily form cyclified conformations. Another type of side-chain - side chain cyclization is the formation of an amide structure between an aspartic acid or glutamic acid residue and the base amino acid, which requires that the side chain protection group must be able to be selectively removed from the polypeptide either on the resin or after dissociation. The third type of side-chain - side chain cyclization is the formation of diphenyl ethers by tyrosine or p-hydroxyphenylglycine. This type of cyclization in natural products is only found in microbial products, and cyclization products often have potential medicinal value. The preparation of these compounds requires unique reaction conditions, so they are not often used in the synthesis of conventional peptides.
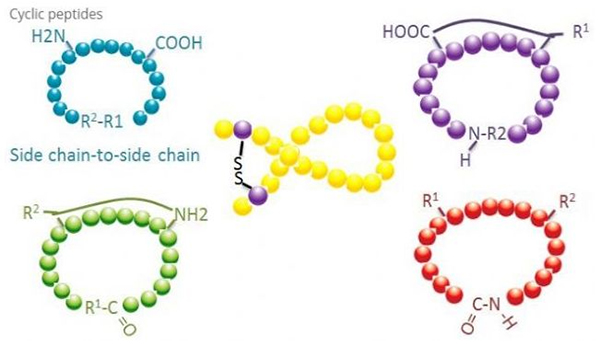
(2) terminal-to-sidechain
Terminal-side chain cyclization usually involves the C-terminal with the amino group of the lysine or ornithine side chain, or the N-terminal with the aspartic acid or glutamic acid side chain. Other polypeptide cyclization is made by forming ether bonds between terminal C and serine or threonine side chains.
(3) Terminal or head-to-tail type
Chain polypeptides can be either cycled in a solvent or fixed on a resin by side chain cyclation. Low concentrations of peptides should be used in solvent centralization to avoid oligomerization of peptides. The yield of a head-to-tail synthetic ring polypeptide depends on the sequence of the chain polypeptide. Therefore, before preparing cyclic peptides on a large scale, a library of possible chained lead peptides should first be created, followed by cyclization to find the sequence with the best results.
2. N-methylation
N-methylation originally occurs in natural peptides and is introduced into peptide synthesis to prevent the formation of hydrogen bonds, thereby making peptides more resistant to biodegradation and clearance. Synthesis of peptides using N-methylated amino acid derivatives is the most important method. In addition, Mitsunobu reaction of N-(2-nitrobenzene sulfonyl chloride) polypeptide-resin intermediates with methanol can also be used. This method has been used to prepare cyclic peptide libraries containing N-methylated amino acids.
3. Phosphorylation
Phosphorylation is one of the most common post-translational modifications in nature. In human cells, more than 30% of proteins are phosphorylated. Phosphorylation, especially reversible phosphorylation, plays an important role in controlling many cellular processes, such as signal transduction, gene expression, cell cycle and cytoskeleton regulation, and apoptosis.
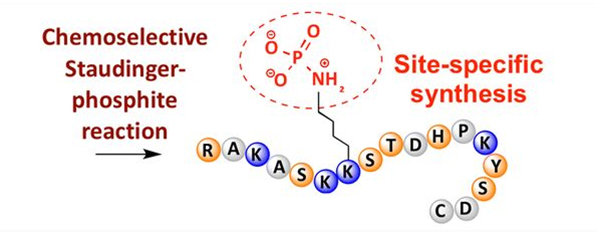
Phosphorylation can be observed at a variety of amino acid residues, but the most common phosphorylation targets are serine, threonine, and tyrosine residues. Phosphotyrosine, phosphothreonine, and phosphoserine derivatives can be either introduced into peptides during synthesis or formed after peptide synthesis. Selective phosphorylation can be achieved using residues of serine, threonine, and tyrosine that selectively remove protective groups. Some phosphorylation reagents can also introduce phosphoric acid groups into the polypeptide by post modification. In recent years, site-specific phosphorylation of lysine has been achieved using a chemically selective Staudinger-phosphite reaction (Figure 3).
4. Myristoylation and palmitoylation
Acylation of the N-terminal with fatty acids allows peptides or proteins to bind to cell membranes. The myridamoylated sequence on the N-terminal enables Src family protein kinases and reverse transcriptase Gaq proteins to be targeted to bind to cell membranes. Myristic acid was linked to the N-terminal of the resin-polypeptide using standard coupling reactions, and the resultant lipopeptide could be dissociated under standard conditions and purified by RP-HPLC.
5. Glycosylation
Glycopeptides such as vancomycin and teicolanin are important antibiotics for the treatment of drug-resistant bacterial infections, and other glycopeptides are often used to stimulate the immune system. In addition, since many microbial antigens are glycosylated, it is of great significance to study glycopeptides for improving the therapeutic effect of infection. On the other hand, it has been found that the proteins on the cell membrane of tumor cells exhibit abnormal glycosylation, which makes glycopeptides play an important role in cancer and tumor immune defense research. Glycopeptides are prepared by Fmoc/t-Bu method. Glycosylated residues, such as threonine and serine, are often introduced into polypeptides by pentafluorophenol ester activated fMOCs to protect glycosylated amino acids.
6. Isoprene
Isopentadienylation occurs on cysteine residues in the side chain near the C-terminal. Protein isoprene can improve cell membrane affinity and form protein-protein interaction. Isopentadienated proteins include tyrosine phosphatase, small GTase, cochaperone molecules, nuclear lamina, and centromeric binding proteins. Isoprene polypeptides can be prepared using isoprene on resins or by introducing cysteine derivatives.
7. Polyethylene glycol (PEG) modification
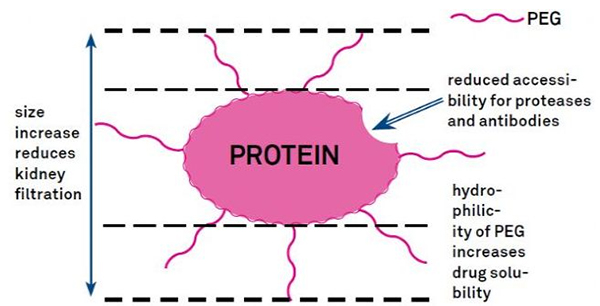
PEG modification can be used to improve protein hydrolytic stability, biodistribution and peptide solubility. The introduction of PEG chains to peptides can improve their pharmacological properties and also inhibit the hydrolysis of peptides by proteolytic enzymes. PEG peptides pass through the glomerular capillary cross section more easily than ordinary peptides, greatly reducing renal clearance. Due to the extended active half-life of PEG peptides in vivo, the normal treatment level can be maintained with lower doses and less frequent peptide drugs. However, PEG modification also has negative effects. Large amounts of PEG prevent the enzyme from degrading the peptide and also reduce the peptide's binding to the target receptor. But PEG peptides' low affinity is usually offset by their longer pharmacokinetic half-life, and by being present in the body longer, PEG peptides have a greater likelihood of being absorbed into target tissues. Therefore, PEG polymer specifications should be optimized for optimal results. On the other hand, PEG peptides accumulate in the liver due to reduced renal clearance, resulting in macromolecular syndrome. Therefore, PEG modifications need to be designed more carefully when peptides are used for drug testing.
Common modification groups of PEG modifiers can be roughly summarized as follows: Amino (-amine) -NH2, aminomethyl-Ch2-NH2, hydroxy-OH, carboxy-Cooh, sulfhydryl (-Thiol) -SH, Maleimide -MAL, succinimide carbonate -SC, succinimide acetate -SCM, succinimide propionate -SPA, n-hydroxysuccinimide -NHS, Acrylate-ch2ch2cooh, aldehyde -CHO (such as propional-ald, butyrALD), acrylic base (-acrylate-acrl), azido-azide, biotinyl -Biotin, Fluorescein, glutaryl -GA, Acrylate Hydrazide, alkyne-alkyne, p-toluenesulfonate -OTs, succinimide succinate -SS, etc. PEG derivatives with carboxylic acids can be coupled to n-terminal amines or lysine side chains. Amino-activated PEG can be coupled to aspartic acid or glutamic acid side chains. Mal-activated PEG can be conjugated to mercaptan of fully deprotected cysteine side chains [11]. PEG modifiers are commonly classified as follows (note: mPEG is methoxy-PEG, CH3O-(CH2CH2O)n-CH2CH2-OH) :
(1) straight chain PEG modifier
mPEG-SC, mPEG-SCM, mPEG-SPA, mPEG-OTs, mPEG-SH, mPEG-ALD, mPEG-butyrALD, mPEG-SS
(2) bifunctional PEG modifier
HCOO-PEG-COOH, NH2-PEG-NH2, OH-PEG-COOH, OH-PEG-NH2, HCl·NH2-PEG-COOH, MAL-PEG-NHS
(3) branching PEG modifier
(mPEG)2-NHS, (mPEG)2-ALD, (mPEG)2-NH2, (mPEG)2-MAL
8. Biotinization
Biotin can be strongly bound with avidin or streptavidin, and the binding strength is even close to covalent bond. Biotin-labeled peptides are commonly used in immunoassay, histocytochemistry, and fluorescence-based flow cytometry. Labeled antibiotin antibodies can also be used to bind biotinylated peptides. Biotin labels are often attached to the lysine side chain or the N terminal. 6-aminocaproic acid is often used as a bond between peptides and biotin. The bond is flexible in binding to the substrate and binds better in the presence of steric hindrance.
9. Fluorescent labeling
Fluorescent labeling can be used to trace polypeptides in living cells and to study enzymes and mechanisms of action. Tryptophan (Trp) is fluorescent, so it can be used for intrinsic labeling. The emission spectrum of tryptophan depends on the peripheral environment and decreases with decreasing solvent polarity, a property that is useful for detecting peptide structure and receptor binding. Tryptophan fluorescence can be quenched by protonated aspartic acid and glutamic acid, which may limit its use. The Dansyl chloride group (Dansyl) is highly fluorescent when bound to an amino group and is often used as a fluorescent label for amino acids or proteins.
Fluorescence resonance Energy conversion (FRET) is useful for enzyme studies. When FRET is applied, the substrate polypeptide usually contains a fluorescence-labeling group and a fluorescence-quenching group. Labeled fluorescent groups are quenched by the quencher through non-photon energy transfer. When the peptide is dissociated from the enzyme in question, the labeling group emits fluorescence.
10. Cage polypeptides
Cage peptides have optically removable protective groups that shield the peptide from binding to the receptor. When exposed to UV radiation, the peptide is activated, restoring its affinity to the receptor. Because this optical activation can be controlled according to time, amplitude, or location, cage peptides can be used to study reactions occurring in cells. The most commonly used protective groups for cage polypeptides are 2-nitrobenzyl groups and their derivatives, which can be introduced in peptide synthesis via protective amino acid derivatives. Amino acid derivatives that have been developed are lysine, cysteine, serine, and tyrosine. Aspartate and glutamate derivatives, however, are not commonly used due to their susceptibility to cyclization during peptide synthesis and dissociation.
11. Polyantigenic peptide (MAP)
Short peptides are usually not immune and must be coupled to carrier proteins to produce antibodies. Polyantigenic peptide (MAP) is composed of multiple identical peptides connected to lysine nuclei, which can specifically express high potency immunogens and can be used to prepare peptide-carrier protein couplets. MAP polypeptides can be synthesized by solid phase synthesis on MAP resin. However, incomplete coupling results in missing or truncated peptide chains on some branches and thus does not exhibit the properties of the original MAP polypeptide. As an alternative, peptides can be prepared and purified separately and then coupled to MAP. The peptide sequence attached to the peptide core is well-defined and easily characterized by mass spectrometry.
Conclusion
Peptide modification is an important means of designing peptides. Chemically modified peptides can not only maintain high biological activity, but also effectively avoid the drawbacks of immunogenicity and toxicity. At the same time, chemical modification can endow peptides with some new excellent properties. In recent years, the method of C-H activation for the post-modification of polypeptides has been rapidly developed, and many important results have been achieved.
Post time: Mar-20-2023
