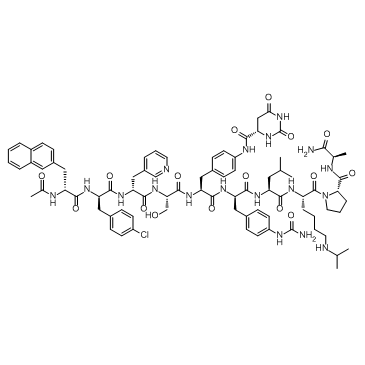
Introduction to Background
Degarelix is a GNRH receptor inhibitor that reversibly inhibits GNRH receptors in the pituitary gland, thereby reducing the release of gonadotropins and further inhibiting the secretion of testosterone. Degarelix may delay the progression and deterioration of prostate cancer by reducing testosterone, which is essential for prostate cancer growth. This drug from the Rongling (Ferring) company was developed on December 24, 2008 and approved by the US Food and Drug Administration (FDA), the trade name is Firmagon.
Currently, degarelix can be synthesized by various methods. However, due to the presence of seven non-natural hydrophobic amino acids in the peptide sequence, Degarelix crude products often contain more by-products and impurities during the synthesis process, which put forward high requirements for the separation and purification process. At present, the isolation and purification process of degarelix crude products is rarely documented in the public literature, and most of them are recrystalized. Therefore, we wished to provide a novel method for degarelix purification.
Methods of purification
1. Dissolution: the degarelitide obtained by solid-phase synthesis method was dissolved in acetic acid aqueous solution, acetonitrile aqueous solution or ethanol aqueous solution.
2. The first purification: octane bonded silica gel was used as column filler, and the mobile phase was divided into A1 phase and B1 phase. The solution obtained in step 1 was washed away by gradient and qualified pure fractions were collected. Then, the qualified pure fraction was diluted at least twofold with aqueous acetic acid, aqueous acetonitrile, or aqueous ethanol to obtain the diluted pure fraction, in which the A1 phase was aqueous acetic acid and the B1 phase was preparation-grade acetonitrile.
3. The second purification: octadecyl bonded silica gel was used as the column filler, and the mobile phase was divided into A2 phase and B2 phase. One pure diluted fraction was washed off by gradient, and two pure qualified fractions were collected. The dichroic qualified fraction was then diluted to more than twice the original volume with aqueous acetic acid, aqueous acetonitrile, or aqueous ethanol to obtain a dichroic diluted fraction. Among them, the A2 phase was trifluoroacetate-triethylamine buffer, and the B2 phase was preparative grade acetonitrile.
4. Third purification: using octadecyl bonded silica gel as column filler, the mobile phase was divided into A3 and B3 phases, the diluted fractions of the second purification were treated with gradient elution, and the fractions that met the third purification standards were collected. Then, three diluted fractions were obtained by diluting at least twice the volume of these standard fractions using aqueous acetic acid, aqueous acetonitrile, or aqueous ethanol. Among them, the A3 phase was ammonium acetate buffer and the B3 phase was preparative grade acetonitrile.
5. Concentrate the three pure diluted fractions in column, and concentrate by rotary evaporation under reduced pressure.
6, the reduced pressure concentrated peptide solution was mixed with an appropriate amount of tert-butyl alcohol aqueous solution, filtered to obtain the filtrate, then freeze-dried, and finally the powdered Garek finished product was obtained.
Post time: Mar-19-2025