The drug of hydrochloric acid XXX is very common, you can search (hydrochlorid) in the Martinale Pharmacopeia, a lot (625 kinds), considering that sometimes only Cl leaves, Chinese or translated into hydrochloric acid XXX, there will be 691 kinds (search chloride). When you search for the online version of traditional Chinese medicine, you will often be prompted that you meet too many requirements to display, so when you go to buy medicine, you often find a lot of hydrochloric acid XXX medicine on the box.
Gutuo explained why hydrochloric acid is added to drugs to make hydrochloric acid, known as “salt” in the pharmaceutical field, that is, two ionic states of the molecules through ionic binding, often can greatly change the physical and chemical properties of the drug.
Change the salt, change the drug
1. Biological activity
The metabolic capacity of the drug (the extent and rate of drug absorption) and toxicity are mainly considered, because in the drug screening stage, it is most important to determine which compound is effective, there are no absorption problems, and toxicity investigations are rare. But in the drug development stage, drug absorption has become an important influencing factor.
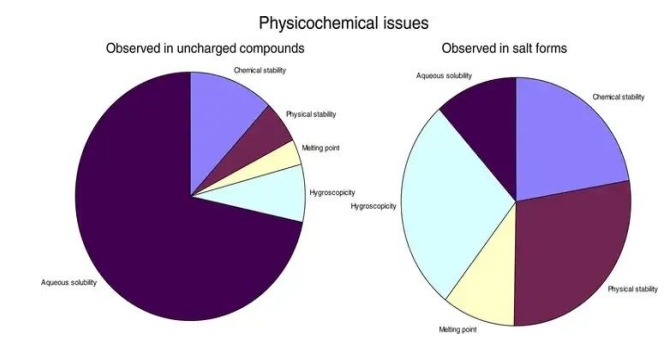
Because the solubility of the hydrochloride in the drug is often very high in various salts, the chloride ion is often not toxic at all (of course, the release rate is too fast to exceed the toxic dose, so the higher the faster the better), so it is often selected, especially for drugs with low solubility. Salts can also alter the microenvironment of drug molecules and alter drug solubility. However, salt is far less simple than simply increasing solubility. The dissolution process after salt is much more complex and you do not know which salt has the highest solubility and the highest biological activity (it is difficult to predict the solubility differences of the various salts).
The following picture is from the PPT of Teacher Tong from Wei-qin Utah University. Suitable for pharmacy professionals.
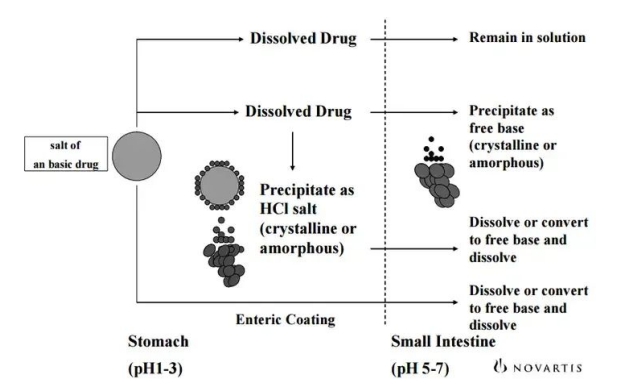
After the salt of an alkaline drug (assuming this is sulfate) is taken and reaches the stomach (pH 1-3, acidic), the dissolved part is absorbed in the stomach and the part continues to be absorbed in the small intestine. Some drugs can bind to gastric acid to produce hydrochloric acid XXX, which precipitates in an acidic environment. There are other possible hydrochlates in the figure that are generated that are encapsulated in the sulfate drug but converted to the drug molecule itself in the small intestine (pH 5-7, neutral) and then absorbed. This process is obviously influenced by many factors (gastric emptying time, gastric pH, etc.).
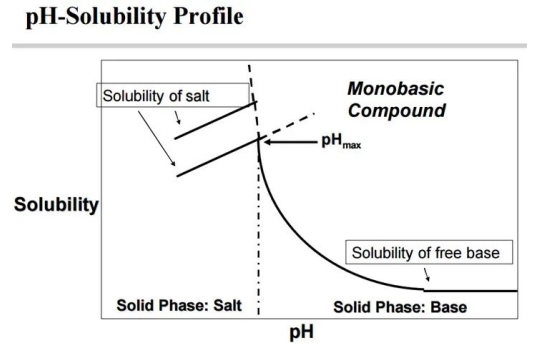
The common solubility curve of HCL can only be measured by being below pHmax. When the pH is large, you only measure the solubility of the alkaline drug, which of course is small.
In addition, the picture also shows that different salts often have different pHmax solubility, which also suggests that you want to distinguish them only by distinguishing their solubility in an acidic environment and simply supplement them here.
2. Physicochemical stability
Some drugs have very low melting points. When they become salt, their melting point increases, so that stability improves (the rate of degradation at room temperature decreases, and the impurity mass decreases). In addition, physical stability is an interesting aspect. Some drugs change in response to changes in their surroundings under solid conditions. An extreme example is carbon, which can be the hardest diamond, or the ultra-soft graphite (polycrystalline cash). The same can happen with drugs. Temperature, humidity, operating conditions, and even occasional impurities during drug production can lead to drastic changes in the arrangement of drug molecules, that is, changes in the crystal form, and different biological activities between the different crystal forms. For example, the example of Ritonavir [1] (skip it if you know). In 1995, Abbott Laboratories (Ya, most people know it is because of milk powder) launched the drug, but after the launch in 1998, it was found that the original drug crystal suddenly did not exist in the factory, and the drug became a new crystal! A large number of dissolution experiments used to test the quality failed, and all drugs had to be recalled until the problem was resolved and put back on the market. It is said to have lost $200 million (not only missed the market, but also suffered huge reputative damage). However, there are few reports on the polycrystal of salt, but salt also has the problem of salt, which tends to be hygroscopicity under high humidity.
3. Easy to scale up production
I think hydrochloric acid is the most advantageous. Hydrochloric acid is cheap and convenient. It’s the easiest thing to do. Just add it. But sometimes some hydrochloric acids may not be prepared and can be prepared by changing the solvent.
Post time: May-14-2024