In the biomedical industry, peptide drugs have attracted much attention due to their high efficiency and low side effects. However, the complexity of the polypeptide structure is essential for a full understanding of its properties and function. As a powerful tool, cross-linked mass spectrometry has provided us invaluable assistance in solving the mystery of peptide structure in complex samples. This article will introduce the cross-linked mass spectrometry technique in detail, from principle to application, and take you into this fascinating field of science.
1. Basic principles of cross-linked mass spectrometry:
Cross-linked mass spectrometry is an important support for mass spectrometry, which is mainly used to study the structure and interaction of protein peptides. The basic principle is to “link” different functional regions in a protein or peptide by a crosslinker to form a crosslinked compound. These cross-linked compounds were subsequently analyzed and tested by a mass spectrometer.
2. Test operation process:
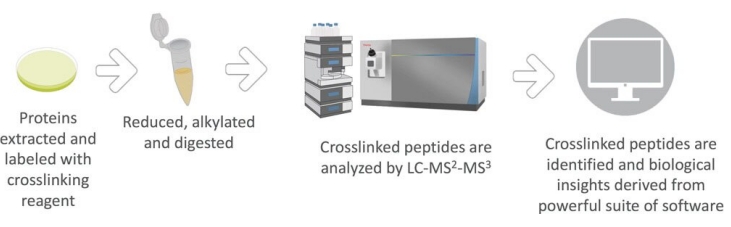
A. Crosslinker selection: The selection of appropriate crosslinker is particularly important for a successful trial. Common cross-linkers include silica, formaldehyde, etc.
b. Cross-linking reaction: The sample is reacted with a cross-linking agent to promote cross-linking between peptides.
c. Enzymatic digestion: To further improve the accuracy of mass spectrometry, the cross-linked compounds must be digested enzymatically and converted into smaller fragments.
d. Mass spectrometry: The enzymatic hydrolyzed samples were injected into the mass spectrometer, and the structural information of the cross-linked compounds was obtained by mass spectrometry.
How can cross-linked mass spectrometry be used to analyze the structure of peptides in complex samples?
3. Application of cross-linking mass spectrometry in peptide structure analysis:
A. Peptide drug structure studies: Cross-linked mass spectrometry provides a reliable method to study the precise structure of peptide drugs. By comparing the mass spectra of cross-linked compounds, scientists can determine the mode of linkage between different amino acid residues in a peptide and then reveal the spatial structure of the peptide.
b. Protein complex analysis: Many biological processes involve the interaction between proteins and other biomolecules. Cross-linked mass spectrometry can help us understand the composition and structure of protein complexes and then further investigate their function and control mechanisms.
c. Disease identification studies: Certain diseases result in pathological expression or structural changes of specific peptides. Cross-linked mass spectrometry can help identify these changes and provide new clues for the diagnosis and treatment of diseases.
4. Challenges and future prospects of cross-linked mass spectrometry:
Although cross-linked mass spectrometry has great potential for peptide structure analysis, it still faces several challenges. Issues such as the complexity of the sample and the interpretation of mass spectrometry analysis for the selection of cross-linkers are continuously improved and refined. In the future, we can expect greater breakthroughs in the resolution, sensitivity and automation of cross-linked mass spectrometry to provide strong support for a wider range of biomedical research.
5. Results
As an important tool in the biomedical field, cross-linked mass spectrometry brings important information to the analysis of peptide structures in complex samples. By understanding the rationale and experimental operation of cross-linked mass spectrometry, we can better understand its application in peptide drug development, protein interaction, and disease marker research. With the continuous development of technology, cross-linked mass spectrometry will continue to play an important role in the biomedical industry, promoting progress and innovation in pharmaceutical science.
Post time: Jan-12-2024