DesmopressinAcetate/16679-58-6/GT Peptide/Peptide Supplier
Description
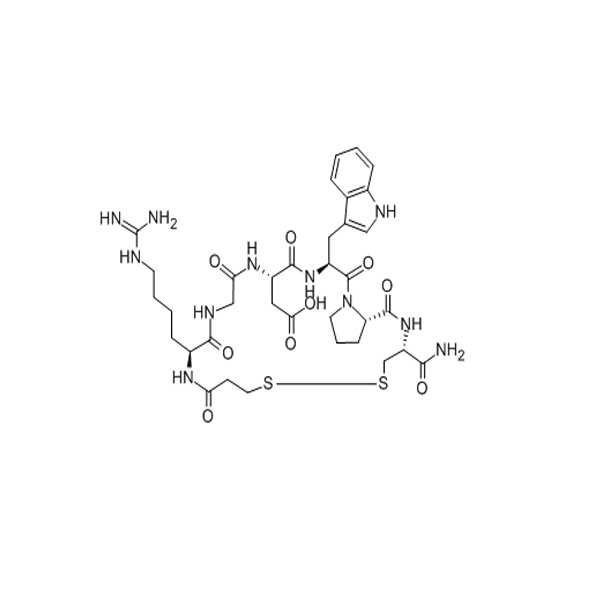
Desmopressin acetate is a newly synthesized vasopressin in recent years. The cysteine at position 1 and the L-arginine at position 8 of vasopressin are replaced by β-thiol propionic acid and D-arginine, respectively. “The pressor effect of this product is 2000 times less than that of vasopressin, while the antidiuretic effect is stronger and longer lasting than that of natural vasopressin, with a half-life of 3 times longer.” The appearance was white powder.
Specifications
Apperance: White to off-white powder
Purity(HPLC): ≥98.0%
Single Impurity: ≤2.0%
Acetate Content(HPLC): 5.0%~12.0%
Water Content (Karl Fischer): ≤10.0%
Peptide Content: ≥80.0%
Packing and Shipping: Low temperature, vacuum packing, accurate to mg as required.
How To Order?
1. Contact us directly by phone or email: +86-13735575465, sales1@gotopbio.com.
2. Order online. Please fill out the order online form.
3. Provide peptide name, CAS No. or sequence, purity and modification if required, quantity, etc. we will provide a quotation within 2 hours.
4. Order conformation by duly signed sales contract and NDA(non disclosure agreement) or confidential agreement.
5. We will continuously update the order progress in time.
6. Peptide delivery by DHL, Fedex or others, and HPLC, MS, COA will be provided along with the cargo.
7. Refund policy will be followed if any discrepancy of our quality or service.
8. After-sale service: If our clients have any questions about our peptide during experiment, please feel free to contact us and we will respond to it in a short time.
All products of the company are only used for scientific research purpose, it’s prohibited to be directly used by any individuals on human body.
FAQ:
Were the peptides containing Cys reduced before shipment?
If the peptide is not found to have been oxidized, we generally do not reduce Cys. All polypeptides are obtained from crude products purified and lyophilized under pH2 conditions, which at least to some extent prevent the oxidation of Cys. Peptides containing Cys are purified at pH2 unless there is a specific reason to purify at pH6.8. If purification is performed at pH6.8, the purified product must be treated with acid immediately to prevent oxidation. In the final quality control step, for the peptides containing Cys, if the presence of molecular weight (2P+H) substance is found on the MS map, it indicates that a dimer has been formed. If there is no problem with MS and HPLC, we will directly lyophilize and ship the goods without any further processing. It should be noted that peptides containing Cys undergo slow oxidation over time, and the degree of oxidation depends on peptide sequence and storage conditions.
How do you determine if a peptide is looped?
We use the Ellman reaction to test whether the ring formation is complete. If the Ellman test is positive (yellow), the ring reaction is incomplete. If the test results are negative (not yellow), the ring reaction has been complete. We do not provide the analysis report of cyclization identification for our clients. Generally, there will be a description of Ellman’s test results in the QC report.
I need a cyclic peptide, which contains a tryptophan, will it be oxidized?
The oxidation of tryptophan is a common phenomenon in peptide oxidation, and peptides are usually cyclized before purification. If the oxidation of tryptophan occurs, the retention time of the peptide on the HPLC column will change, and the oxidation can be removed by purification. Furthermore, oxidized peptides can also be detected by MS.
Is it necessary to put a gap between the peptide and the dye?
If you are going to attach a large molecule (such as a dye) to the peptide, it is best to put a space between the peptide and the ligand to minimize interference with the receptor by the folding of the peptide itself or by the folding of its conjugate. Others do not want intervals. For example, in the folding of proteins, it is possible to determine how far apart the folding structure of an amino acid is by attaching a fluorescent dye to a particular site.
If you want to do a biotin modification at the N terminal, do you need to put a gap between the biotin and the peptide sequence?
The standard biotin labeling procedure used by our company is to attach an Ahx to the peptide chain, followed by biotin. Ahx is a 6-carbon compound that acts as a barrier between the peptide and the biotin.
Can you give some advice on the design of phosphorylated peptides?
As the length increases, the binding efficiency gradually decreases from the phosphorylated amino acid onwards. The synthesis direction is from the C terminal to the N terminal. It is recommended that the residues after the phosphorylated amino acid should not exceed 10, that is, the number of amino acid residues before the phosphorylated amino acid from the N terminal to the C terminal should not exceed 10.
Why the n-terminal acetylation and C-terminal amidation?
These modifications prevent the peptide from being degraded and allow the peptide to mimic its original state of alpha amino and carboxyl groups in the parent protein.